Categories
Sterile osteomyelitis in the ulnar diaphysis of a young indoor cat
Domenico Sainato (https://www.eastcottreferrals.co.uk/the-team/domenico-sainato/)
Filippo Cinti (www.eastcottreferrals.co.uk/member/24/Filippo-Cinti/)
Helen Renfrew (https://www.eastcottreferrals.co.uk/the-team/helen-renfrew/)
First Published February 3, 2020 – https://doi.org/10.1177/2055116919899754
Case summary
A 3-year-old neutered male indoor British Shorthair cat was referred for a 2-week history of intermittent right forelimb lameness. Radiographic examination showed a diaphyseal monostotic, expansile, fusiform, lytic lesion in the right ulna. CT further defined the lesion and also demonstrated ipsilateral pulmonary consolidation. Histology was conclusive of osteomyelitis, and microbiology and fluorescence in situ hybridisation analysis (FISH) were negative on aerobic and anaerobic bacterial culture, as well as fungal culture. Clinical and radiographic improvement was seen after anti-inflammatory treatment and a short initial period of antibiosis.
Relevance and novel information
This is an unusual monostotic diaphyseal cortical location for osteomyelitis in cats and, moreover, may represent a rare case of sterile osteomyelitis. To our knowledge, non-traumatic osteomyelitis in this location in cats has not been reported in the veterinary literature.
Case description
A 3-year-old neutered male indoor British Shorthair cat was referred to our hospital for a 2-week history of intermittent lameness on the right forelimb. No recent or past trauma was reported.
On physical examination the patient was bright and responsive, with a good body condition score, normal vital parameters, temperature and chest auscultation. A painful swelling of the right antebrachium, with no evidence of a bite wound, was observed. The area was not hot and the range of motion of the carpus, elbow and shoulder was normal. Examination of the forelimbs and hindlimbs was otherwise unremarkable. Results from haematology and serum biochemical analysis showed no significant abnormalities.
Under general anaesthesia, mediolateral and craniocaudal radiographs of the left and right antebrachium and manus were obtained using a digital radiography system (ACEM Vet Care Raffaello HF30-HF3-/4; Konica Minolta Regius Σ II [54 kVp, 20 mAs]) and a table-top technique.
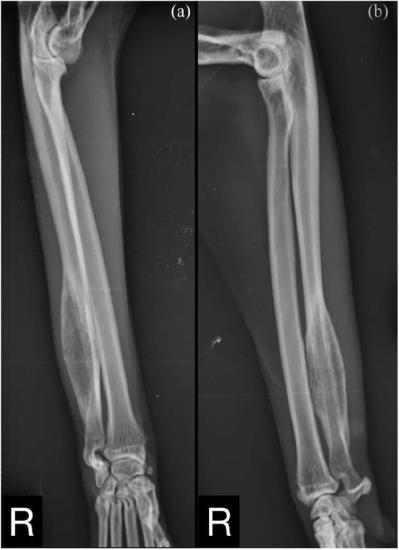
Radiological evaluation revealed moderate swelling of the soft tissue around the radius and ulna, and a large monostotic ulnar lesion measuring approximatively 40 × 10 mm at the level of the distal diaphysis (Figure 1). This lesion extended distally to the distal ulnar metaphysis, and proximally along the ulnar diaphysis for approximately 40% of its length. There was a short transitional zone between the lesion and the normal bone.
Figure 1 (a) Craniocaudal and (b) mediolateral views of the right antebrachium. A large monostotic lesion is observed on the distal diaphysis of the ulna
The mass had a fusiform shape, with evidence of moth-eaten lysis and cortical expansion. A focal solid periosteal reaction could be seen on the lateral margin of the proximal aspect of the lesion, while a small area of lamellated periosteal reaction was noted on the medial margin. A triangular area of organised subperiosteal new bone (Codman’s triangle) was also evident.
Based on the radiographic findings, our differential diagnoses were primary bone tumour, secondary bone tumour, osteomyelitis and bone cyst (eg, aneurysmal bone cyst). To better characterise the mass and rule out pulmonary metastasis, a CT scan of the forelimbs and thorax was performed. The scan was carried out under general anaesthesia using a four-slice CT scanner (GE LightSpeed; General Electric Healthcare). The patient was positioned in sternal recumbency with the forelimbs extended and parallel to each other. On the CT workstation, images were acquired with soft tissue, pulmonary and bone algorithms, and reformatted in sagittal and dorsal planes. The images were reviewed using a DICOM viewer application (Horos, version 3.3.0; GNU Lesser General Public License) using soft tissue (Window Width [WW] = 400, Window Level [WL] = 40), pulmonary (WW = 1400, WL = −500) and bone windows (WW = 1500, WL = 300). Adjustments to image window width and level were made as needed.
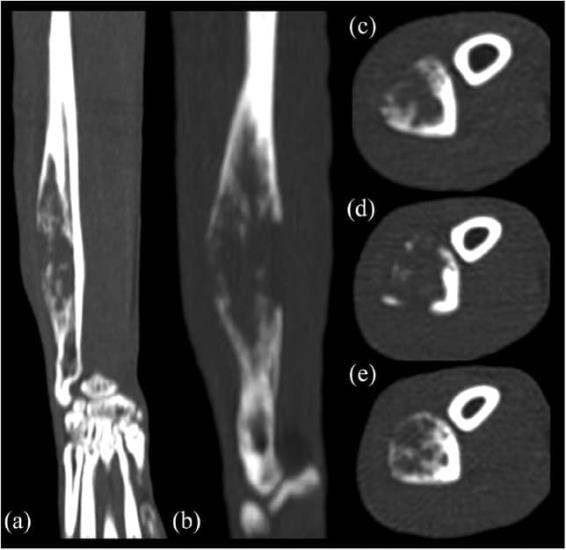
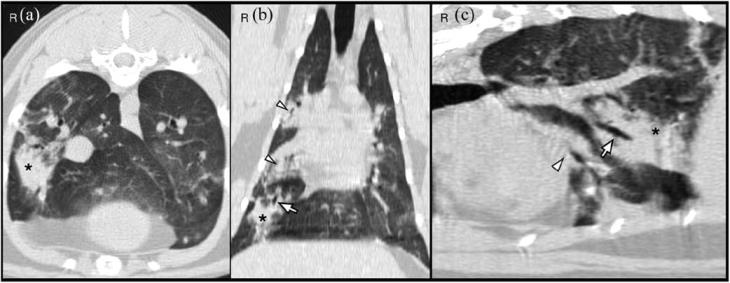
The CT images showed a monostotic, diaphyseal, fusiform, expansile, highly lytic mass involving the cortex of the ulna, sparing only the medial aspect and measuring 42 × 11 × 9 mm (Figure 2). There was no evidence of sequestrum formation. There was moderate ground-glass opacity and mild bronchial wall thickening affecting the right middle, right caudal, right accessory and left caudal pulmonary lobes. There were four areas of hyperdense alveolar filling observed in the right pulmonary lobes (Figure 3). These areas were fairly well defined and highly attenuating; they were localised to the dorsal and middle aspect of the right caudal lobe, ventral aspect of the right middle lobe and caudal portion of the right cranial lobe. Some of the bronchi involved in these areas of consolidation were truncated. No lymphadenomegaly was evident. Differentials for the pulmonary findings were multifocal infectious disease, haemorrhage and pulmonary metastatic disease (less likely). Atelectasis was ruled out because the areas of hyperattenuation were in a non-dependent position, and both lungs were optimally inflated and symmetrically expanded.
Figure 2 (a) Dorsal, (b) sagittal and (c–e) transverse multiplanar CT reformatted images of the right antebrachium. Cross-sectional imaging confirms the features of the ulnar lesion and better highlights the severity of the geographical lytic pattern
Figure 3 (a) Transverse, (b) dorsal and (c) lesion-orientated oblique sagittal multiplanar CT reformatted images of the thorax. A focal area of pulmonary consolidation is visualised in the right caudal lobe (*). There is a truncated bronchus within the lesion (arrow). Other areas of pulmonary hyperattenuation can be seen on the right cranial and right middle pulmonary lobes (arrowheads)
Based on these findings, a presumptive diagnosis of incidental, multifocal, chronic bronchopneumonia with atypical haematogenous ulnar osteomyelitis was made. A primary or a secondary bone tumour was considered less likely.
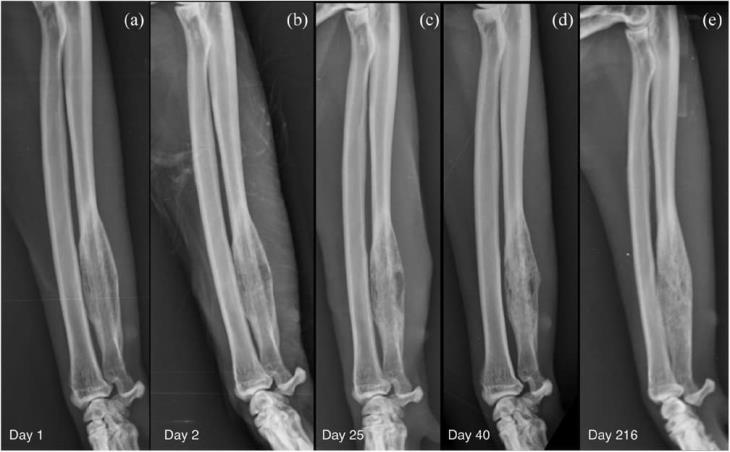
Multiple bone biopsies were performed using a 14 G Jamshidi needle (T-Lok; Argo). Two samples were taken from the centre and two from the periphery of the lesion. Post-biopsy radiographs of the right antebrachium showed no evidence of iatrogenic fractures. Based on the presumption of a bacterial disease process, a 2-week course of amoxicillin–clavulanate (Clavamax; Beecham) 20 mg/kg PO q12h was started along with a 7-day course of meloxicam (Metacam; Boehringer) 0.1 mg/kg PO q24h. Subsequent histological diagnosis was consistent with multifocal, marked, neutrophilic and lymphocytic osteomyelitis. Microbiology was negative for aerobic and anaerobic culture, fungal culture and mycobacteria. Furthermore, fluorescence in situ hybridisation (FISH) analysis did not detect any bacteria. No further antibiotics were prescribed. Following the initial presentation (Figure 4a), radiographic follow-up was performed at day 25 (Figure 4b) and day 40 (Figure 4c) from diagnosis (5 and 20 days after the end of the course of antibiotics). An increase in radiopacity of the lytic lesion was evident due to in-filling with bone on day 40 (Figure 4d). A further increase in radiopacity was noticed at a long-term radiographic follow-up performed at day 216 (Figure 4e). At this time clinical signs had resolved, with the absence of lameness and pain on palpation of the right forelimb.
Figure 4 Sequential mediolateral radiographs of the right antebrachium performed at (a) day 1 (day of presentation), (b) day 2 (after bone biopsy), (c) day 25 (5 days after the end of the initial course of antibiotics), (d) day 40 (20 days after the end of the initial course of antibiotics), (e) day 216 (long-term follow-up). The radiograph performed after the bone biopsy (b) shows a defined area of radiolucency in the centre of the lesion representing the bone deficit at the biopsy site. An increasing opacity within the lesion with a less moth-eaten appearance of the medulla, smoothing of the caudal and cranial bony contours and merging of the periosteal new bone with the cortex on follow-up radiography represents bone production as healing takes place (c–e); however, bone remodelling at the long-term follow-up was slower than expected.
Follow-up thoracic imaging was performed radiographically at day 40. A left lateral view showed resolution of the abnormal alveolar filling previously detected in the right lung lobes.
Discussion
Inflammatory (infectious and non-infectious) and neoplastic bone lesions tend to show similar marked radiographic changes, and histopathology and microbiological culture need to be performed to reach a definitive diagnosis. Assessing the aggressiveness of a bone lesion is fundamental and systematic evaluation of the radiographs should be performed in order to assess the pattern of bone lysis and bone production, the zone of transition, the periosteal reaction, the cortical response, the rate of change and any extraosseous extension.1 However, clinicians should be aware of the low sensitivity and specificity of this imaging modality in the diagnosis of osteomyelitis.2 In this case, a pattern of geographical lysis associated with a narrow transition zone, solid and lamellated periosteal reaction, thinning and expansion of the cortex were suggestive of a less aggressive, slow-growing lesion.3
Owing to the unusual presentation of the lesion, both primary or metastatic neoplastic disease were considered. Osteosarcoma is the most common primary bone tumour in cats; it is primarily osteolytic,4 slow to metastasise5 and usually affects the metaphysis of long bones. Because slow-growing and low-grade malignant osteosarcomas (eg, parosteal osteosarcoma) are reported,6 failure to visualise pulmonary metastasis or having a diaphyseal location, as in this case, does not exclude them as differentials. The presence of the Codman’s triangle was not considered relevant for the diagnosis. While in the past this was considered to be pathognomonic for osteosarcoma, it is now reported as non-specific as it can result from any rapid periosteal elevation owing to neoplastic, infectious or traumatic disease.7
As the lesion was diaphyseal in location, it could also represent a long bone metastatic disease.8 Other differentials that were considered were metastasis from an unknown primary neoplasm9 and osteoclastoma. The latter is a benign bone neoplasm affecting the long bones, especially the distal ulna. Radiographically it is an expansile, lytic lesion but usually metaphyseal and not painful.
Histological results were consistent with marked, multifocal, neutrophilic and lymphocytic osteomyelitis. Bone infections are usually polyostotic if the dissemination is haematogenous, but direct inoculation is more common and leads to monostotic lesions that can be non-metaphyseal.10 In this case, however, no bite wound or fracture was found and no history of trauma or recent surgery was provided. Also, although a diaphyseal location for osteomyelitis has been reported in old dogs, to our knowledge this is the first time that a diaphyseal osteomyelitis has been reported in a young cat without evidence of direct inoculation or iatrogenic cause.
Sampling of the pulmonary lesion either by fine-needle aspiration or bronchoalveolar lavage would have been interesting in the light of the suspected sterile osteomyelitis, and would have aided diagnosis and treatment.
At the time, financial constraints did not allow further procedures to be undertaken. As the cat was asymptomatic for its respiratory lesions, the bone biopsy took precedence. Chronic non-bacterial osteomyelitis is a rare auto-inflammatory bone disorder affecting children that presents with bone pain arising from sterile osteomyelitis. The disease is focal and metaphyseal plates of the long bones are the most commonly affected site. The lesion is lytic during the early phase, with sclerosis and bony expansion during later stages. Common histological findings include acute and/or chronic inflammation, marrow fibrosis or normal bone; it is a diagnosis of exclusion. Non-steroidal anti-inflammatory drugs are used as the first-line treatment. If after 3 months the treatment fails, a second-line treatment is needed.11 Sterile osteomyelitis is a rare condition in veterinary medicine and it is described as the result of a reaction to surgical implants that have been permanently left in bones.12
As the CT was suggestive of pneumonia, an atypical, rare, monostotic haematogenous osteomyelitis spreading to the ulna from a chronic pulmonary infection, or the reverse, was considered as the primary differential diagnosis. As no pathogen was detected by microbiology culture and FISH analysis, a presumptive diagnosis of sterile osteomyelitis was subsequently made. The possibility that bacterial growth may be inhibited by antibiotic therapy prior to biopsy was excluded, since no antibiotic treatment had been administered to the patient before or at the time of biopsy. Nevertheless, a definitive pathogenesis for the bone lesion could not be determined because of some limitations. These included: the lack of abdominal imaging in order to search more fully for an alternative focus of infection or primary neoplasia; the possibility that the patient had a small wound or abscess not noticed by the owner that had completely healed by the time of presentation; or due to factors related to the sample collection or handling. Also, the neutrophilic aggregates observed in the tissue sampled from the bone lesion did not help in establishing the pathogenesis, as they could have been primarily due to a bacterial infection or secondary to tissue necrosis of any cause (eg, ischaemia, cyst degeneration, local trauma). It might be that this case could have a similar cause as the previously reported case of scapular osteomyelitis in a young cat.13
As the lameness completely resolved, the owner declined further investigations. From our point of view, further imaging would have been ideal to document ongoing healing and to identify any deterioration in case of ongoing disease. However, where there are no clinical signs, further imaging requiring sedation and using ionising radiation is not ethical. Our follow-up ended at 7 months, and while healing was occurring, the bone was not yet normal. The previously reported case of scapular osteomyelitis showed near resolution of the bony expansion after 8 months. As the bone in this case was still markedly abnormal after 7 months, it was on a trajectory to take far longer for the bone contour to return to normal. The reason for the slow healing is not clear, but could involve a suboptimal local blood supply, or other metabolic disease (of which there was no evidence).
It is also possible that there was ongoing slowly resolving disease such as true sterile osteomyelitis.
A short course of antibiotics would be very unlikely to result in control of the disease if it had been a bacterial osteomyelitis. In traumatic osteomyelitis in dogs, the rate of recurrent infection is markedly diminished when antibiotic treatment continues for more than 1 month after wound healing,14 and in this case only a fortnight’s course was prescribed.
Conclusions
This is a unique case of feline osteomyelitis in a diaphyseal location in a young, indoor cat with no history of trauma. It was not possible to establish the pathogenesis of the process, but given the lack of pathogens detected by microbiology culture and FISH analysis, a sterile process is a possibility.
Acknowledgements
We thank Katherine Gray Berman DVM, DACVP, MRCVS for the written assistance with the histopathological interpretation of the submitted samples.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
This work involved the use of non-experimental animals only (owned or unowned), and followed internationally recognised high standards (‘best practice’) of individual veterinary clinical patient care. Ethical approval from a committee was not therefore needed.
Informed consent
Informed consent (verbal or written) was obtained from the owner or legal custodian of all animals described in this work for the procedures undertaken. No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
References
| 1. | O’Donnell, P . Evaluation of focal bone lesions: basic principles and clinical scenarios. Imaging 2003; 15: 298–323. Google Scholar | Crossref |
| 2. | Bubenik, L . Infections of the skeletal system. Vet Clin Small Anim Pract 2005; 35: 1093–1109. Google Scholar | Crossref | Medline |
| 3. | Kirberger, R . Bones – general. In: Kirberger, RM, McEvoy, FJ (eds). Canine and feline musculoskeletal imaging. 2nd ed. Gloucester: BSAVA, 2016, pp 75–86. Google Scholar | Crossref |
| 4. | Turrell, J, Pool, RR. Primary bone tumors in the cat: a retrospective study of 15 cats and a literature review. Vet Radiol Ultrasound 1982; 23: 152–166. Google Scholar | Crossref |
| 5. | Chun, R . Common malignant musculoskeletal neoplasms of dogs and cats. Vet Clin North Am Small Anim Pract 2005; 35: 1155–1167. Google Scholar | Crossref | Medline | ISI |
| 6. | Thomas, W, Daniel, GW, McGavin, MD. Parosteal osteosarcoma of the cervical vertebra of a dog. Vet Radiol Ultrasound 1997; 38: 120–123. Google Scholar | Crossref | Medline |
| 7. | Mirra, JM . Osseous tumors of intramedullary origin. In: Mirra, JM (ed). Bone tumors: clinical, radiologic and pathologic correlations. Philadelphia, PA: Lea & Febiger, 1989, p 270. Google Scholar |
| 8. | Dennis, R, Kirberger, RM, Wrigley, RH, et al. Skeletal system: general. In: Dennis, R, Kirberger, RM, Wrigley, RH, et al (eds). Handbook of small animal radiology and ultrasound. 2nd ed. St Louis, MO: Saunders, 2010, pp 1–30. Google Scholar | Crossref |
| 9. | Moore, G . Osteosarcoma in adjacent lumbar vertebrae in a dog. J Am Vet Med Assoc 2000; 217: 1038–1040. Google Scholar | Crossref | Medline | ISI |
| 10. | Thrall, D . Radiographic features of bone tumors and bone infections in dogs and cats. In: Thrall, D (ed). Textbook of veterinary diagnostic radiology. 7th ed. St Louis, MO: Saunders, 2018, pp 392–396. Google Scholar | Crossref |
| 11. | Zhao, Y, Ferguson, P. Chronic nonbacterial osteomyelitis and chronic recurrent multifocal osteomyelitis in children. Pediatr Clin North Am 2018; 65: 783–800. Google Scholar | Crossref | Medline |
| 12. | McAllister, H, Tobin, E. Long bones – mature. In: Kirberger, RM, McEvoy, FJ (eds). Canine and feline musculoskeletal imaging. 2nd ed. Gloucester: BSAVA, 2016, pp 108–132. Google Scholar | Crossref |
| 13. | Viskjer, S, Rapp, M. Scapular osteomyelitis in an immature domestic shorthair cat. JFMS Open Rep 2016; 2. DOI: 10.1177/2055116916668199 Google Scholar | SAGE Journals |
| 14. | Nunamaker, DM . Osteomyelitis. In: Newton, CD, Nunamaker, DM (eds). Textbook of small animal orthopaedics. Philadelphia, PA: J.B. Lippincott Company, 1985, p 37. Google Scholar |